Post by Nadica (She/Her) on Nov 15, 2024 2:59:23 GMT
HICPAC Is Finally Revisiting Critical Isolation Recommendations Reversed Earlier This Year - Published Nov 14, 2024
By Don Ford
When you ask? Right now.
Just like the last rush job, this action has a short shelf life.
This goes out the morning of the 14th with the event on the 14th and 15th.
We don’t need a lot of people to write letters, we just need enough.
If you are a part of the COVID Conscious Community (CCC) then you have undoubtedly heard about HICPAC.
In Nov 2023, there was a big push to reverse a decision for the official CDC recommendation to include both surgical masks and respirators offering equal protection.
It was an all hands on deck moment for the community, and it wasn’t just a single event where we expressed outrage; it included a 60-day comment period as well.
We were ultimately successful, with the CDC sending back the guidelines.
We have continued our activism, pushing on HICPAC for preferable guidance.
And over the next two days they meet to finally revisit these recommendations that haven’t been changed since 2007…
But according to the 2007 documents the last change was brought on by the original SARS from 2003, but they were unable to lock in the best practices.
We ignored aerosol transmission once, and it set us up for our most recent failure.
This is not only a decision that will right the ship now but also correct the mistake that had been left to history.
But what is the guideline and what exactly is happening?
More importantly… How can you help?
t’s exactly what the headline describes, so no reason to waste any time.
With the meeting actually today, I’m going to explain it as quickly as possible.
Then there is a call to action similar to the one I organized for the last VRBPAC.
Workshops happen on Thursday and voting is on Friday… but…
What is HICPAC?
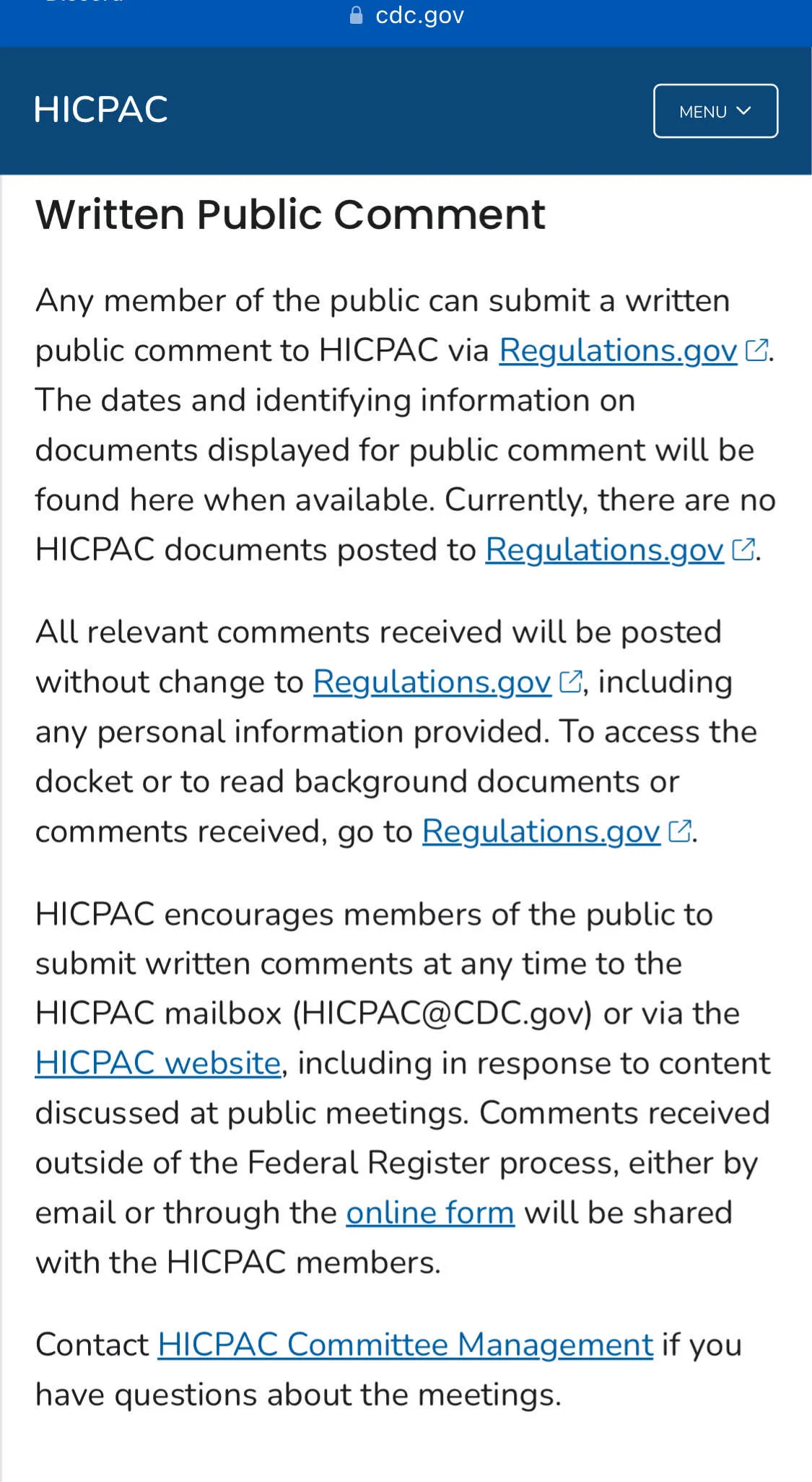
HICPAC is one of a few federal agencies where we organize public comment campaigns regarding matters of public health. These are hosted on Regulations.gov and much of the comment process is automated through it.
This time the federal document is up, but something is specifically missing:
There is no public comment tied directly to Regulations.gov.
They instead want you to send it to their email HICPAC@cdc.gov.
NNU are also asking you to cc them: healthandsafety@nationalnursesunited.org.
Don’t worry about tracking those emails right now, I’ll have them again at the end.
But the concern is that public comment pushes are effective because we are going on the official record that can later be looked at by anyone.
It becomes a permanent US record.
That process is regulated by “The Federal Advisory Committee Act.”
But HICPAC seems to run by slightly different rules. They seem to only feel required to hold public comment AFTER the work groups have already met and come to a recommendation.
Then after 60 days of public comment, the CDC can choose to accept that recommendation or not.
That was the “multi-month” letter writing comment mentioned earlier where the CDC ultimately DID NOT accept their recommendation.
Now, they can do public comment before the meeting just like last year, and we did get an email back saying they might do it…
But for some reason, instead of setting up an automated process, they are instead accepting comments manually, and I can’t help but feel that’s fishy behavior.
Especially after last year when they only allowed public comment for a single day.
Their own website explains it perfectly well.
Before you are completely enraged by this, I need you to remember something…
It’s important to distinguish the difference between the whole committee who organizes the event and materials with the 10 (should be 14) voting members.
The voting members are not the ones taking part in this questionable behavior.
The call-to-action involves contacting those voting members, so remember… we are not using this space to air those grievances.
Instead, please send them to HICPAC@cdc.gov.
And definitely send them.
This is a great opportunity to push for change, but sometimes change can almost sneak up on us… happening when we least expect it.
Just like the change we got right after the last vote on this issue.
Why have things changed since the last vote?
At least one major thing shifted after the CDC kicked back the recommendations.
For years the perceived concept that disease was primarily spread through droplets instead of more fine aerosol has limited our ability to protect people.
And the WHO just completely cleaned the slate moving everything that travels through the air to exactly that… I had a pretty serious rant on our show about a week before they suddenly changed it because what else would it travel through at any point other than the air?.. Are we mer-people?
I’m sure the two are unrelated.
What are the actual questions being asked?
These questions were generated by the CDC blog when they kicked back the HICPAC recommendations on masking protections.
The blog is actually really worth a read if you have the time.
But the questions are specifically related to required infection control and though there are two work groups, it’s an issue that covers both groups.
This isn’t actually four questions, this is actually nine questions.
Should there be a category of Transmission-based Precautions that includes masks (instead of NIOSH Approved® N95® [or higher-level] respirators) for pathogens that spread by the air?
No. Not in the current format of standardization. The only acceptable lower level than an N95 would be NIOSH approved KN95 that allows for ear loops but that does not currently exist in a standardized form.
Should N95 respirators be recommended for all pathogens that spread by the air?
No, some pathogens that spread through the air need greater protection.
Can the work-group clarify the criteria that would be used to determine which transmission by air category applies for a pathogen?
The optimal criteria would be a separation into wet and dry categories.
“Wet” would include micro-aerosol, aerosol, heavy laden (droplets), and *ahem* fecal aerosols; all of which are airborne to different levels traveling in different sized water particles to their destination. The size of the actual pathogen determines what scale of airborne it needs to infect hosts.
“Dry” would be dust primarily but also include some fomites. Reminder, fomites are the items the viral particles in the air settle on, not the particles themselves. These would be primarily limited to pathogens that can sustain in dry states like pox viruses.
For the category of Special Air Precautions, can you clarify if this category includes only new or emerging pathogens or if this category might also include other pathogens that are more established?
This should include existing pathogens. With the “death” to what is known as “droplet theory” we need to assume that these changes expand to all existing pathogens, not simply novel ones.
Can you also clarify what constitutes a severe illness?
Any illness that creates long term quality of life issues. We think of disease as simply the acute phase but many folks have asymptomatic acute phases, no symptoms at all, but go on to experience disability still. The recognition of severity should not be limited to whether someone is hospitalized or not.
Is the current guideline language sufficient to allow for voluntary use of a NIOSH Approved N95 (or higher-level) respirator?
No. The current regulations are already interpreted as greater respirator protection is seen as unnecessary. Employers are already forcing employees to remove their respirators citing fitting rules intended to protect employees as limitations to whether they can mask voluntarily at all. Forcing them to remove their mask entirely rather than wear what might be perceived as an ill fitting respirator.
Should the document include a recommendation about healthcare organizations allowing voluntary use?
The recommendation should be endorsing respirator use in general.
Should there be a recommendation for use of source control in healthcare settings that is broader than current draft recommendations?
Yes, the last recommendations failed to secure protections in 2007 that were already needed in 2003. Other countries adapted but we failed, leaving our people at risk. Healthcare should be a place of healing, not a place people are afraid to go to out of fear they might die from an HAI.
Should source control be recommended at all times in healthcare facilities?
At all times.
In order to make spaces safe there won’t be a single protection (masks, ventilation, UVC sterilization, etc) that will ultimately get the job done.
It will be all of those protections working in conjunction with each other.
But rather than simply demand it all be sorted out in one meeting…
HICPAC needs to establish work-groups to sort out specific details, which should include, but not be limited to…
Best uses of UVC.
Create a timeline for hospitals to update their air filtration systems.
Establishing better building standards for new healthcare facilities.
Setting shorter timelines for updates to these recommendations.
Filling in the other 4 spots with expertise in the above roles.
Seeking a NIOSH approved KN-95 style ear loop respirator for ease of use.
Different quality masks for different situations.
There is a lot of talk of “N95 or greater” around this committee, and we helped frame that talking point on our Brace for Impact show in 2022.
I only say that last bit because I’ve experienced an unusual response…
We run into a small problem if the phrasing is interpreted too literally.
If we say the requirement is N95 or greater then we will only get N95s.
While we can always enable the use of higher quality masks, that still puts the onus on the worker when the requirement should be on the employers or institutions.
With the emergence of H5N1 and other pathogens making a comeback, we need to make sure employers are protecting their workforce, which needs to include a variety of types of masks from N95s to P100 elastomerics.
Instead of approaching this as a minimum requirement, it should be viewed as a scaling issue requiring different respirators for different situations so that hospitals don’t get caught without the proper PPE available in an emergency situation.
This PPE being available is no different than having a fire extinguisher on the wall.
And with wildfires on the rise, the standardization of the oil based protection P type respirators provide should not be outside the purview of this conversation.
While there is some concern of resistance from hospitals and providers, once SARS-CoV-2 is added to the HAC list, which limits Medicare and Medicaid payouts and based on the changes to WHO policy this is the natural progression, then healthcare providers will be eager to update their ventilation, get respirators on their workforce, and invest in UVC.
As almost all hospitals make a large portion of their revenue from Medicare and Medicaid patients, establishing a higher base level of care from that perspective creates better care for all patients.
Once their ability to collect government payments has been cut in half, the private equity who have purchased these facilities will have no problem making the necessary investments to give a modern-level quality of care.
But adoption among healthcare workers?
Adoption of respirator use is going to happen based on options.
Having more options for respiratory protection gives the illusion of choice, and instead of having a choice between respiratory protection and no respiratory protection… Different types of protection should be regulated to be available so they can be choosing between preferable types of protection instead of no protection at all.
Once the advancements in institutional disease control have been made (filtration, UVC, sterilization), a NIOSH approved KN-95 style mask that has simple ear loops but a fit testable seal should be a priority for ease of use for medical workers.
This will ultimately decrease the resistance to adoption.
We should probably throw in a wage increase too… but I digress.
How can you help?
This is a tough one.
We have a rare environment creating a pressure cooker for ideas and not all of them are going to be automatically good.
There are new members to HICPAC this year, walking into this mess…
And some folks who have been around for a half of their session already.
The main problem being that with public comment not being handled properly and empty promises on materials being delivered to members…
We have to sidestep those impediments to make contact with the voting members.
We need to contact them and try to have reasonable conversations about the decisions being made here.
There are a few groups who have put out their ideas about what should be prioritized, and they are all certainly worth a read, if you have the time.
National Nurses United has been pushing hard on this issue more than anyone.
Nurses → act.nnu.org/sign/submit-cdc-hicpac-feedback
WHN → whn.global/submit-written-public-comments-for-hicpac-meeting-on-november-14-15/
WHN 4 questions → whn.global/whn-response-to-four-cdc-questions-on-preventing-transmission-in-healthcare-settings/
And let’s not forget the current guidelines → www.cdc.gov/infection-control/hcp/isolation-precautions/index.html
But what we are going to do is contact the members about all of these issues… and I’ll be honest, I don’t want you to send an exact form…
Write a letter that is personal to you using whichever part of this article or any of the others that struck you as important.
And if all else fails, just send this article with a polite hello.
We don’t want to waste their time but we do want to get their attention.
And above all else, be nice.
Here are your contacts.
Katherine Ellingson: kellingson@arizona.edu
Lela Luper: lela.luper@chickasaw.net
Lisa Baum: Lisa.baum@nysna.org
Laura Evans: leevans@uw.edu
Erica Shenoy: eshenoy@mgh.harvard.edu
Connie Steed: csteed@ghs.org
David Jay Weber: dweber1@email.unc.edu
Colleen Kraft: colleen.kraft@emory.edu
Jennie H. Kwon: j.kwon@wustl.edu
Sharon Wright: sbwright@bidmc.harvard.edu
Plus the additional email I promised in the article itself…
HICPAC@cdc.gov.
CC the NUU: healthandsafety@nationalnursesunited.org.
(Follow the link for more pertainent links)
By Don Ford
When you ask? Right now.
Just like the last rush job, this action has a short shelf life.
This goes out the morning of the 14th with the event on the 14th and 15th.
We don’t need a lot of people to write letters, we just need enough.
If you are a part of the COVID Conscious Community (CCC) then you have undoubtedly heard about HICPAC.
In Nov 2023, there was a big push to reverse a decision for the official CDC recommendation to include both surgical masks and respirators offering equal protection.
It was an all hands on deck moment for the community, and it wasn’t just a single event where we expressed outrage; it included a 60-day comment period as well.
We were ultimately successful, with the CDC sending back the guidelines.
We have continued our activism, pushing on HICPAC for preferable guidance.
And over the next two days they meet to finally revisit these recommendations that haven’t been changed since 2007…
But according to the 2007 documents the last change was brought on by the original SARS from 2003, but they were unable to lock in the best practices.
We ignored aerosol transmission once, and it set us up for our most recent failure.
This is not only a decision that will right the ship now but also correct the mistake that had been left to history.
But what is the guideline and what exactly is happening?
More importantly… How can you help?
t’s exactly what the headline describes, so no reason to waste any time.
With the meeting actually today, I’m going to explain it as quickly as possible.
Then there is a call to action similar to the one I organized for the last VRBPAC.
Workshops happen on Thursday and voting is on Friday… but…
What is HICPAC?
HICPAC is one of a few federal agencies where we organize public comment campaigns regarding matters of public health. These are hosted on Regulations.gov and much of the comment process is automated through it.
This time the federal document is up, but something is specifically missing:
There is no public comment tied directly to Regulations.gov.
They instead want you to send it to their email HICPAC@cdc.gov.
NNU are also asking you to cc them: healthandsafety@nationalnursesunited.org.
Don’t worry about tracking those emails right now, I’ll have them again at the end.
But the concern is that public comment pushes are effective because we are going on the official record that can later be looked at by anyone.
It becomes a permanent US record.
That process is regulated by “The Federal Advisory Committee Act.”
But HICPAC seems to run by slightly different rules. They seem to only feel required to hold public comment AFTER the work groups have already met and come to a recommendation.
Then after 60 days of public comment, the CDC can choose to accept that recommendation or not.
That was the “multi-month” letter writing comment mentioned earlier where the CDC ultimately DID NOT accept their recommendation.
Now, they can do public comment before the meeting just like last year, and we did get an email back saying they might do it…
But for some reason, instead of setting up an automated process, they are instead accepting comments manually, and I can’t help but feel that’s fishy behavior.
Especially after last year when they only allowed public comment for a single day.
Their own website explains it perfectly well.

Before you are completely enraged by this, I need you to remember something…
It’s important to distinguish the difference between the whole committee who organizes the event and materials with the 10 (should be 14) voting members.
The voting members are not the ones taking part in this questionable behavior.
The call-to-action involves contacting those voting members, so remember… we are not using this space to air those grievances.
Instead, please send them to HICPAC@cdc.gov.
And definitely send them.
This is a great opportunity to push for change, but sometimes change can almost sneak up on us… happening when we least expect it.
Just like the change we got right after the last vote on this issue.
Why have things changed since the last vote?
At least one major thing shifted after the CDC kicked back the recommendations.
For years the perceived concept that disease was primarily spread through droplets instead of more fine aerosol has limited our ability to protect people.
And the WHO just completely cleaned the slate moving everything that travels through the air to exactly that… I had a pretty serious rant on our show about a week before they suddenly changed it because what else would it travel through at any point other than the air?.. Are we mer-people?
I’m sure the two are unrelated.
What are the actual questions being asked?
These questions were generated by the CDC blog when they kicked back the HICPAC recommendations on masking protections.
The blog is actually really worth a read if you have the time.
But the questions are specifically related to required infection control and though there are two work groups, it’s an issue that covers both groups.
This isn’t actually four questions, this is actually nine questions.
Should there be a category of Transmission-based Precautions that includes masks (instead of NIOSH Approved® N95® [or higher-level] respirators) for pathogens that spread by the air?
No. Not in the current format of standardization. The only acceptable lower level than an N95 would be NIOSH approved KN95 that allows for ear loops but that does not currently exist in a standardized form.
Should N95 respirators be recommended for all pathogens that spread by the air?
No, some pathogens that spread through the air need greater protection.
Can the work-group clarify the criteria that would be used to determine which transmission by air category applies for a pathogen?
The optimal criteria would be a separation into wet and dry categories.
“Wet” would include micro-aerosol, aerosol, heavy laden (droplets), and *ahem* fecal aerosols; all of which are airborne to different levels traveling in different sized water particles to their destination. The size of the actual pathogen determines what scale of airborne it needs to infect hosts.
“Dry” would be dust primarily but also include some fomites. Reminder, fomites are the items the viral particles in the air settle on, not the particles themselves. These would be primarily limited to pathogens that can sustain in dry states like pox viruses.
For the category of Special Air Precautions, can you clarify if this category includes only new or emerging pathogens or if this category might also include other pathogens that are more established?
This should include existing pathogens. With the “death” to what is known as “droplet theory” we need to assume that these changes expand to all existing pathogens, not simply novel ones.
Can you also clarify what constitutes a severe illness?
Any illness that creates long term quality of life issues. We think of disease as simply the acute phase but many folks have asymptomatic acute phases, no symptoms at all, but go on to experience disability still. The recognition of severity should not be limited to whether someone is hospitalized or not.
Is the current guideline language sufficient to allow for voluntary use of a NIOSH Approved N95 (or higher-level) respirator?
No. The current regulations are already interpreted as greater respirator protection is seen as unnecessary. Employers are already forcing employees to remove their respirators citing fitting rules intended to protect employees as limitations to whether they can mask voluntarily at all. Forcing them to remove their mask entirely rather than wear what might be perceived as an ill fitting respirator.
Should the document include a recommendation about healthcare organizations allowing voluntary use?
The recommendation should be endorsing respirator use in general.
Should there be a recommendation for use of source control in healthcare settings that is broader than current draft recommendations?
Yes, the last recommendations failed to secure protections in 2007 that were already needed in 2003. Other countries adapted but we failed, leaving our people at risk. Healthcare should be a place of healing, not a place people are afraid to go to out of fear they might die from an HAI.
Should source control be recommended at all times in healthcare facilities?
At all times.
In order to make spaces safe there won’t be a single protection (masks, ventilation, UVC sterilization, etc) that will ultimately get the job done.
It will be all of those protections working in conjunction with each other.
But rather than simply demand it all be sorted out in one meeting…
HICPAC needs to establish work-groups to sort out specific details, which should include, but not be limited to…
Best uses of UVC.
Create a timeline for hospitals to update their air filtration systems.
Establishing better building standards for new healthcare facilities.
Setting shorter timelines for updates to these recommendations.
Filling in the other 4 spots with expertise in the above roles.
Seeking a NIOSH approved KN-95 style ear loop respirator for ease of use.
Different quality masks for different situations.
There is a lot of talk of “N95 or greater” around this committee, and we helped frame that talking point on our Brace for Impact show in 2022.
I only say that last bit because I’ve experienced an unusual response…
We run into a small problem if the phrasing is interpreted too literally.
If we say the requirement is N95 or greater then we will only get N95s.
While we can always enable the use of higher quality masks, that still puts the onus on the worker when the requirement should be on the employers or institutions.
With the emergence of H5N1 and other pathogens making a comeback, we need to make sure employers are protecting their workforce, which needs to include a variety of types of masks from N95s to P100 elastomerics.
Instead of approaching this as a minimum requirement, it should be viewed as a scaling issue requiring different respirators for different situations so that hospitals don’t get caught without the proper PPE available in an emergency situation.
This PPE being available is no different than having a fire extinguisher on the wall.
And with wildfires on the rise, the standardization of the oil based protection P type respirators provide should not be outside the purview of this conversation.
While there is some concern of resistance from hospitals and providers, once SARS-CoV-2 is added to the HAC list, which limits Medicare and Medicaid payouts and based on the changes to WHO policy this is the natural progression, then healthcare providers will be eager to update their ventilation, get respirators on their workforce, and invest in UVC.
As almost all hospitals make a large portion of their revenue from Medicare and Medicaid patients, establishing a higher base level of care from that perspective creates better care for all patients.
Once their ability to collect government payments has been cut in half, the private equity who have purchased these facilities will have no problem making the necessary investments to give a modern-level quality of care.
But adoption among healthcare workers?
Adoption of respirator use is going to happen based on options.
Having more options for respiratory protection gives the illusion of choice, and instead of having a choice between respiratory protection and no respiratory protection… Different types of protection should be regulated to be available so they can be choosing between preferable types of protection instead of no protection at all.
Once the advancements in institutional disease control have been made (filtration, UVC, sterilization), a NIOSH approved KN-95 style mask that has simple ear loops but a fit testable seal should be a priority for ease of use for medical workers.
This will ultimately decrease the resistance to adoption.
We should probably throw in a wage increase too… but I digress.
How can you help?
This is a tough one.
We have a rare environment creating a pressure cooker for ideas and not all of them are going to be automatically good.
There are new members to HICPAC this year, walking into this mess…
And some folks who have been around for a half of their session already.
The main problem being that with public comment not being handled properly and empty promises on materials being delivered to members…
We have to sidestep those impediments to make contact with the voting members.
We need to contact them and try to have reasonable conversations about the decisions being made here.
There are a few groups who have put out their ideas about what should be prioritized, and they are all certainly worth a read, if you have the time.
National Nurses United has been pushing hard on this issue more than anyone.
Nurses → act.nnu.org/sign/submit-cdc-hicpac-feedback
WHN → whn.global/submit-written-public-comments-for-hicpac-meeting-on-november-14-15/
WHN 4 questions → whn.global/whn-response-to-four-cdc-questions-on-preventing-transmission-in-healthcare-settings/
And let’s not forget the current guidelines → www.cdc.gov/infection-control/hcp/isolation-precautions/index.html
But what we are going to do is contact the members about all of these issues… and I’ll be honest, I don’t want you to send an exact form…
Write a letter that is personal to you using whichever part of this article or any of the others that struck you as important.
And if all else fails, just send this article with a polite hello.
We don’t want to waste their time but we do want to get their attention.
And above all else, be nice.
Here are your contacts.
Katherine Ellingson: kellingson@arizona.edu
Lela Luper: lela.luper@chickasaw.net
Lisa Baum: Lisa.baum@nysna.org
Laura Evans: leevans@uw.edu
Erica Shenoy: eshenoy@mgh.harvard.edu
Connie Steed: csteed@ghs.org
David Jay Weber: dweber1@email.unc.edu
Colleen Kraft: colleen.kraft@emory.edu
Jennie H. Kwon: j.kwon@wustl.edu
Sharon Wright: sbwright@bidmc.harvard.edu
Plus the additional email I promised in the article itself…
HICPAC@cdc.gov.
CC the NUU: healthandsafety@nationalnursesunited.org.
(Follow the link for more pertainent links)

